Research
Research Interests
My research interests are to understand the molecular interactions which regulate high expression of type I collagen in fibrosis and to use this knowledge to discover novel antifibrotic drugs.
Significance
Fibrosis is defined by excessive accumulation of type I collagen in various organs. Type I collagen is heterotrimeric protein composed of two α1(I) and one α2(I) polypeptides which fold into triple helix. This is followed by secretion and polymerization of the protein into fibrils, while the deposition of fibrils within internal organs causes scarring and loss of function. It has been estimated that 45% of population is affected by some form of fibrosis. Hepatic fibrosis is the most common, but lungs, heart, kidneys and skin are also commonly affected. Fibroproliferative diseases are chronic, progressive and usually irreversible, however, there are no approved antifibrotic drugs. Therefore, discovery of chemical compounds that can specifically inhibit excessive type I collagen production in fibrosis is of utmost importance.
Innovation
My lab has pioneered the research on translational regulation of collagen expression. Coordinated translation of collagen mRNAs is an important mechanism to increase type I collagen production in fibrosis. Translation of type I collagen mRNAs is regulated by a unique cis-acting element found in their 5’ UTR; the 5’ stem-loop (5’SL). We cloned LARP6 as the protein which binds 5’ SL with high affinity and specificity. Knock down of LARP6 decreases synthesis of type I collagen by cultured cells. Mutation of 5’SL in the collagen α1(I) gene of mice renders these animals resistant to liver fibrosis. These discoveries suggested that the LARP6 dependent mechanism of type I collagen synthesis is crucial for fibrosis development and prompted the search for inhibitors of LARP6/5’ SL binding.
Premise
Our premise is that it is possible to directly and specifically inhibit excessive type I collagen production in fibrosis by inhibiting binding of LARP5 to 5’SL. This is possible because type I collagen is regulated differently in fibrosis than in normal conditions; in fibrosis its production is stimulated by binding of LARP6 to the 5’SL. In an animal model, disruption of LARP6/5’SL interaction resulted in resistance to hepatic fibrosis, suggesting the relevance of the mechanism in vivo. Therefore, inhibitors of LARP6 dependent type I collagen synthesis will be novel and specific class of antifibrotic drugs.
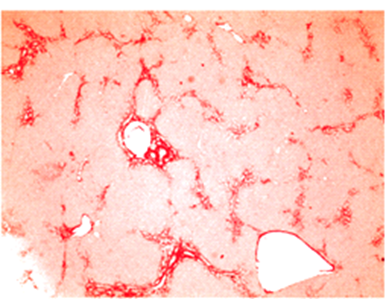
Fibrotic liver: type I collagen staining
Research projects
Most new therapeutics in the last 10 years have been discovered using high throughput phenotypic screens. However, no phenotypic assay that can directly measure type I collagen synthesis has been developed. To make this methodology possible I have devised a collagen biosensor that can recapitulate, visualize and quantify formation of type I collagen in live cells. In addition, alternative technologies for phenotypic screening for type I collagen inhibitors are being developed.
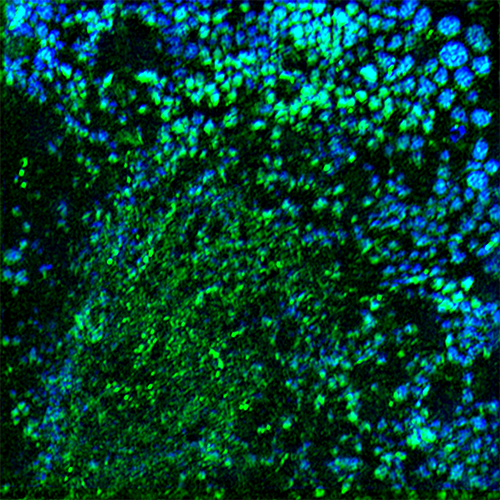
High resolution image of collagenosomes
Projects:
1. Validation of collagen biosensor technology as novel tool for discovery of antifibrotic drugs.We have constructed genes expressing fluorescently labeled collagen polypeptides; these polypeptides fold into heterotrimeric type I collagen and visualize the process in live cells (collagen biosensor). The biosensor revealed that type I collagen is not synthesized uniformly throughout the endoplasmic reticulum, but in discrete granules or bodies, termed collagenosomes. Formation of collagenosomes is dependent on binding of LARP6 to collagen mRNAs and is the hallmark of profibrotic synthesis. Collagen biosensor allows quantification of collagenosomes and can reveal conversion from profibrotic into constitutive synthesis. This project will use high content imaging of cells expressing the biosensor and mathematical image recognition and computer vision to quantify the collagenosomes. This will be the basis for high throughput screens of chemical libraries for inhibitors of collagenosome formation and high level of type I collagen synthesis.
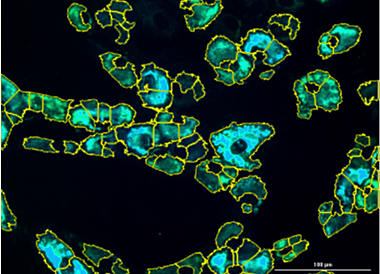
High throughput image of cells expressing the biosensor
2. Design and construction of bimolecular fluorescence complementation (BIFC) system for detection of LARP6 binding.This project is different from collagen biosensor, as it will create a system to monitor LARP6 binding to 5’stem-loop RNA in vivo, rather than monitoring type I collagen formation. The system will be designed such that the fluorescence of LARP6-GFP fusion protein will be activated only upon binding of the protein to the 5’ stem-loop RNA. Thus, by measuring fluorescence of live cells, the bound or free state of LARP6 will be reported. The goal is to establish a reliable system to use in screening for inhibitors of LARP6 binding.
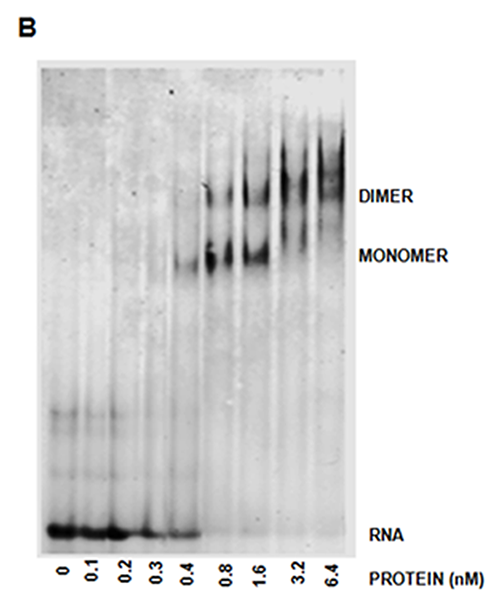
Biochemical determination of LARP6 binding to collagen mRNA
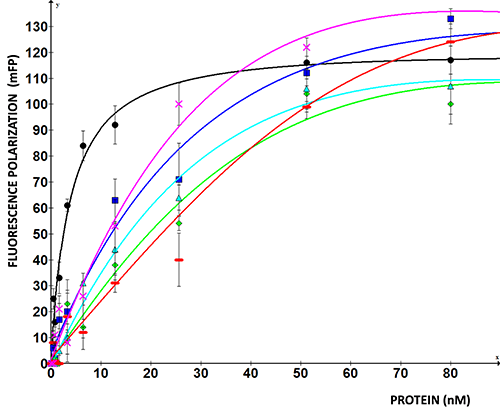
Fluorescence polarization of LARP6
binding
3. Development of reverse yeast three hybrid system for phenotypic screening.This project will engineer yeast strains that can report binding of LARP6 to 5’SL RNA. The strains will express human LARP6 and 5’ stem-loop RNA, but they will grow only when LARP6 is dissociated from the 5’ SL RNA. When LARP6 is bound to 5’SL RNA, the cells will be unable to grow and will serve as reporters of LARP6 dissociation. Inhibitors of LARP6 binding will be screened using this positive phenotypic selection.
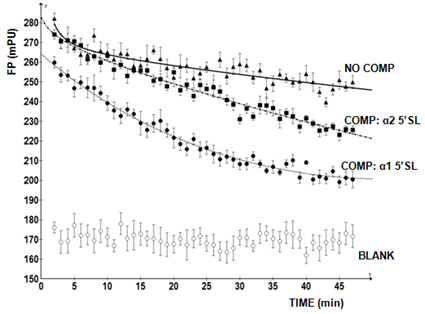
Dissociation rate of LARP6 as basis for screening for antifibrotic drugs
4. Elucidation of LARP6 phosphorylation in fibrosis. Expression of LARP6 does not change much in fibrosis, instead the protein is activated by phosphorylation. TGFβ as the most potent profibrotic cytokine increases phosphorylation of LARP6 on Ser 396. We have raised a phospho-Ser 396 antibody and the project is to characterize this antibody and investigate the pattern of LARP6 phosphorylation in fibrosis as potential diagnostic or prognostic marker.


