Chicken Proteomics
Original Publication: Sakano H, Zorio DAR, Wang X, Ting YS, Noble WS, MacCoss MJ, Rubel EW, Wang Y (2017). Proteomic analyses of nucleus laminaris identified candidate targets of the fragile X mental retardation protein. J Comp Neurol. 525(15):3341-3359. PDF
Animals
This study was performed on White Leghorn chick hatchlings ( Gallus gallus; post-hatch day 0-4). The first sample type was collected specifically from the NL cell group under a laser micro-dissection microscope (LMD-6000; Leica Microsystems). The second type of tissue sample was collected from the dorsal brainstem at the caudorostral level of NL. Protein preparation was performed, followed by trypsin digestion and peptide purification for mass spectrometry.
Mass spectrometry (MS)
The LTQ-FT Ultra (ThermoFisher Scientific) mass spectrometer was used. Spectra were matched to peptide sequences using SEQUEST. Peptide-spectrum match and peptide identifications were obtained from Percolator (v2.01). Peptides with Percolator with q-value <0.01 were given as input to ID Picker for protein identification. We used a decoy database using scrambled Gallus gallus genome sequence (build 12/17/11). We required at least 2 peptides per protein, each with a q -value (false discovery rate) of <0.01. At least four biological replicates with three technical replicates each were performed. We required each peptide to present in every technical replicate (n=3) and at least 2 peptides per protein for identification.
Several software programs were used to perform gene ontology analyses of the identified proteins. The first is the DAVID Bioinformatics Resources 6.8 ( https://david.ncifcrf.gov/ ). We used this resource for protein functional annotation and gene ID conversion. Ensembl Bio-mart software ( http://www.ensembl.org/biomart/martview/ ) was also used for gene ID conversion. For identifying transmembrane proteins, we used the TMHMM program version 2.0 located at: http://www.cbs.dtu.dk/services/TMHMM/ . Finally, we used the Ingenuity pathway analysis at http://www.ingenuity.com/ as an alternative approach to DAVID for identifying enriched pathways.
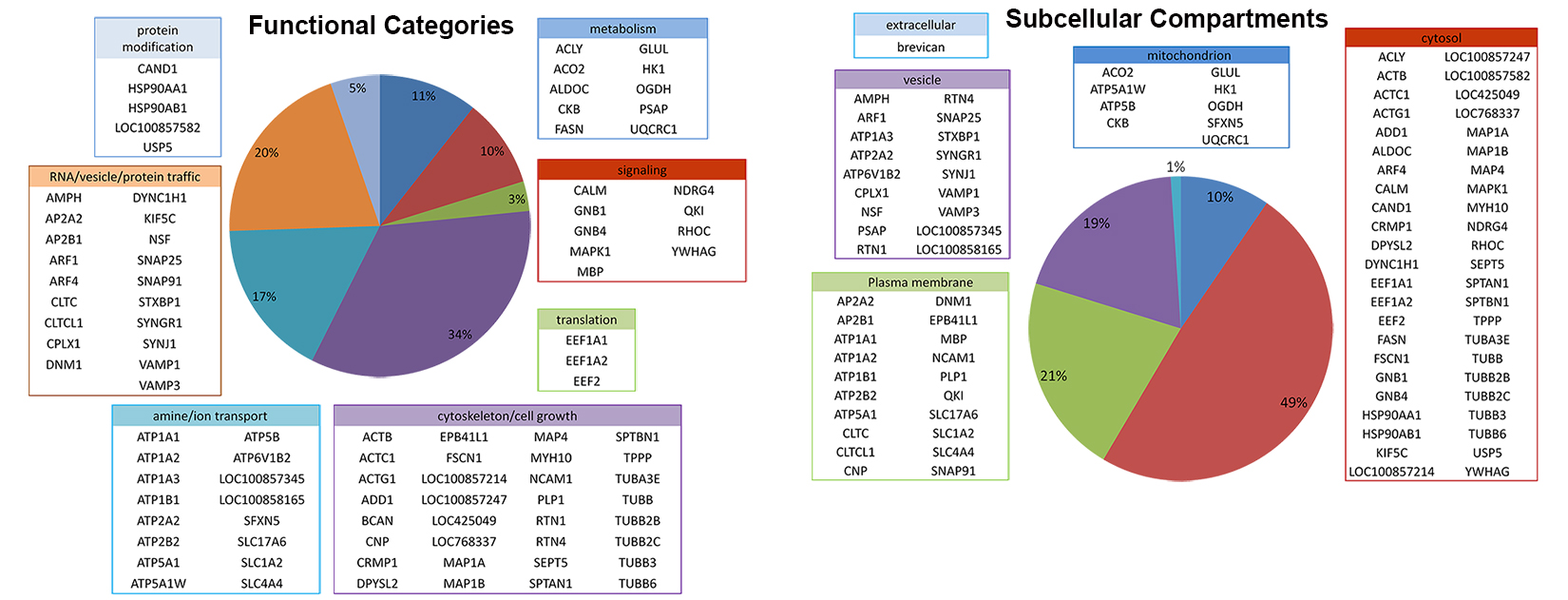
Download: Identified Proteins in NL samples Identified Protein in BS samples (dorsal brainstem)
Putative FMRP targets
Comparative analyses with FMRP targets in the mouse brain download the table of 94 proteins
Thirty-two (32) of the 94 FMRP targets likely to be translated locally download the table of 32 proteins
Cell Growth/Cytoskeleton
CRMP1
collapsin response mediator protein 1
MAP1B
microtubule-associated protein 1B
ACTG1
Actin, gamma 1
SPTBN1
spectrin, beta, non-erythrocytic 1
TUBB
tubulin, beta class I
TUBB2C
tubulin, beta 2C
TUBB2B
tubulin, beta 2B class IIb
TUBB3
tubulin, beta 3 class III
Transport of Ions/Amines (Integral membrane proteins)
ATP6V1B2 (VATB)
ATPase, H+ transporting, lysosomal 56/58kDa, V1 subunit B2
ATP2A2 (SERCA2)
ATPase, Ca++ transporting, cardiac muscle, slow twitch 2
ATP1B1
ATPase, Na+/K+ transporting, beta 1 polypeptide
ATP1A1
ATPase, Na+/K+ transporting, alpha 1 polypeptide
LOC100857345
V-type proton ATPase subunit d 1-like
LOC100858165
V-type proton ATPase subunit d 1-like
Metabolism
HK1
hexokinase 1
ACO2
aconitase 2, mitochondrial
Protein Modification
USP5
ubiquitin specific peptidase 5 (isopeptidase T)
Trafficking of RNA, proteins or vesicles
KIF5C
kinesin family member 5C
DYNC1H1
dynein, cytoplasmic 1, heavy chain 1
DNM1
dynamin 1
ARF1
ADP-ribosylation factor 1
AMPH
amphiphysin
SNAP25
synaptosomal-associated protein, 25kDa
SYNJ1
synaptojanin 1
NSF
N-ethylmaleimide-sensitive factor
SNAP91
synaptosomal-associated protein, 91kDa homolog (mouse)
Signaling
CALM
calmodulin 2 (phosphorylase kinase, delta)
MBP
myelin basic protein
NDRG4
N-myc downstream regulated gene family member 4
Translation
EEF2
eukaryotic translation elongation factor 2
EEF1A1
eukaryotic translation elongation factor 1 alpha 1
EEF1A2
eukaryotic translation elongation factor 1 alpha 2


